
Acid Rain and
Our Nation’s Capital
A Guide to Effects on Buildings and Monuments
by Elaine McGee
For sale by the U.S. Government Printing Office
Superintendent of Documents, Mail Stop: SSOP, Washington, DC 20402-9328
ISBN 0-16-048068-X

Marble surfaces exposed to rain develop a rough “sugary” texture because the calcite grains areloosened as the edges dissolve in the rain water. Column capital volute, Jefferson Memorial,Washington, D.C.

A summer rain storm in Washington, D. C. (Memorial Continental Hall)
When polluted air mixes with rain, snow, and fog, acidprecipitation forms. This acidity has caused people to worry about the environment; some reports showthat acid rain has affected lakes, trees, and fish populations in the Northeastern United Statesand Canada. Another concern is its effect on historic buildings and monuments.
The booklet focuses on acid rain and its impact on our Nation’s capital. Rain in Washington, D. C.,has an average acidity of 4.2, about as acid as a carbonated drink and more than ten times as acid asclean, unpolluted rain. This booklet will define acid rain, explain what effects it has on marble andlimestone buildings, and show, on a walking tour, some of the places in our Nation’s capitalwhere you can see the impact of acid precipitation.
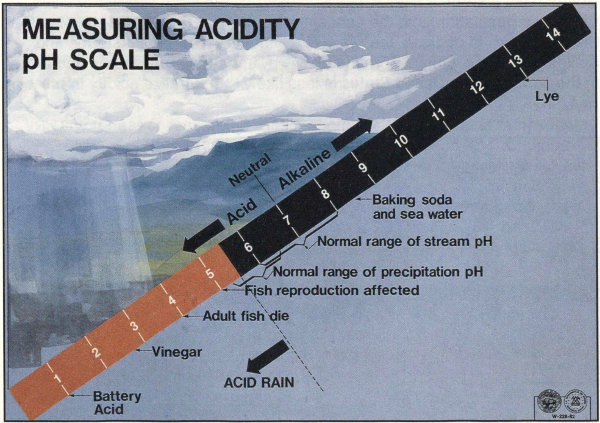
The pH scale: pH = 7 is neutral, neither acid or alkaline; smaller pH values are acid,larger pH values are alkaline. A liquid with a pH of 3 is ten times as acid as one with a pH of 4.
- 1 Battery Acid
- 2.8 Vinegar
- 4 Adult fish die
- <5.5 ACID RAIN
- 5.2-6.5 Normal range of precipitation
- 6-8 Normal range of stream pH
- <7 Acid
- 7 Neutral
- >7 Alkaline
- 8.6 Baking soda and sea water
- 13 Lye
What is acid rain?
The term “acid rain” is commonly used to mean the deposition of acidiccomponents in rain, snow, fog, dew, or dry particles. The more accurateterm is “acid precipitation.” Distilled water, which contains no carbon dioxide,has a neutral pH of 7. Liquids with a pH less than 7 are acid, and those witha pH greater than 7 are alkaline (or basic). “Clean” or unpolluted rain has aslightly acidic pH of 5.6, because carbon dioxide and water in the air react togetherto form carbonic acid, a weak acid. Around Washington, D.C., ho